A Scientific Paper by AlumierMD
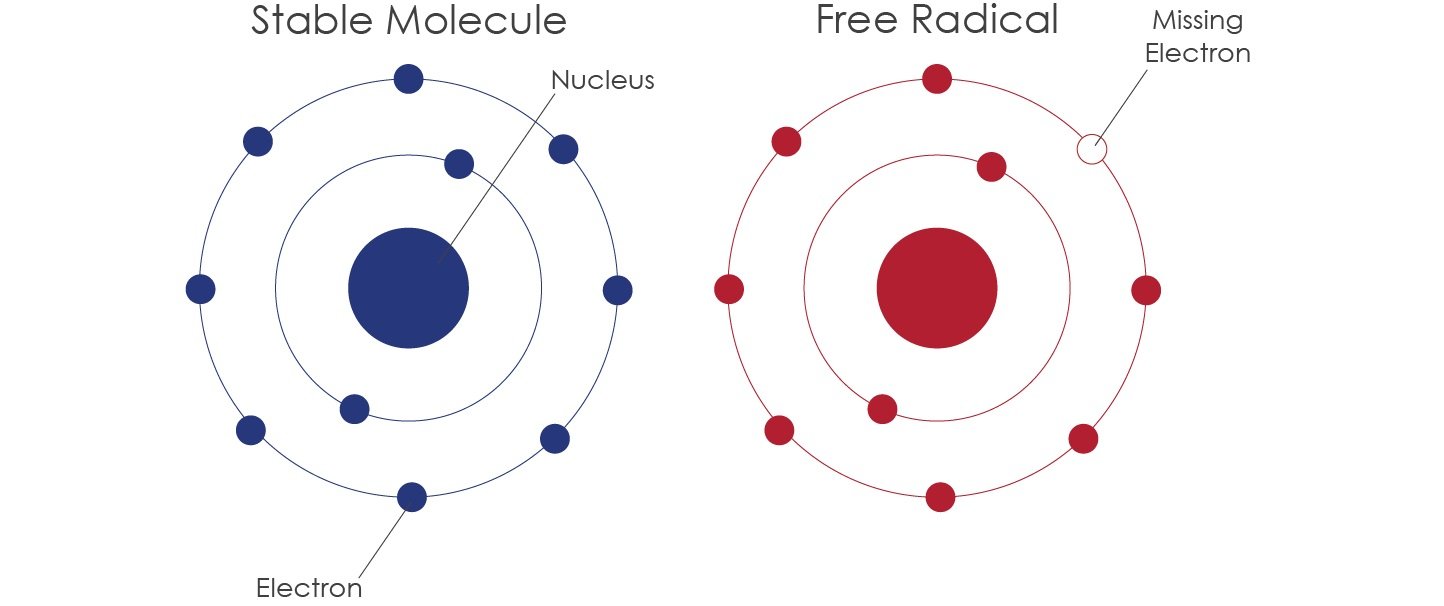
A free radical is an atom or molecule that has an unpaired electron in its outer shell making it unstable and generally highly reactive.
An atom is stable when its outermost shell is full. Because atoms naturally seek to reach a state of stability, an atom will try to fill or empty its outer shell by gaining or losing electrons. Alternatively, atoms can complete their outer shells by sharing electrons with other atoms. By sharing electrons the atoms are bound together to create a stable molecule.
In short, if free radicals are present on your skin they will steal electrons from your healthy skin cells and cause them to develop oxidative stress (which we will discuss below) which then causes premature ageing.How do free radicals form?
While there are different types of free radicals, the most common in aerobic organisms are oxygen free radicals, often referred to as reactive oxygen species (ROS), which include superoxides, hydroxyl anions, hydrogen peroxide and singlet oxygen.
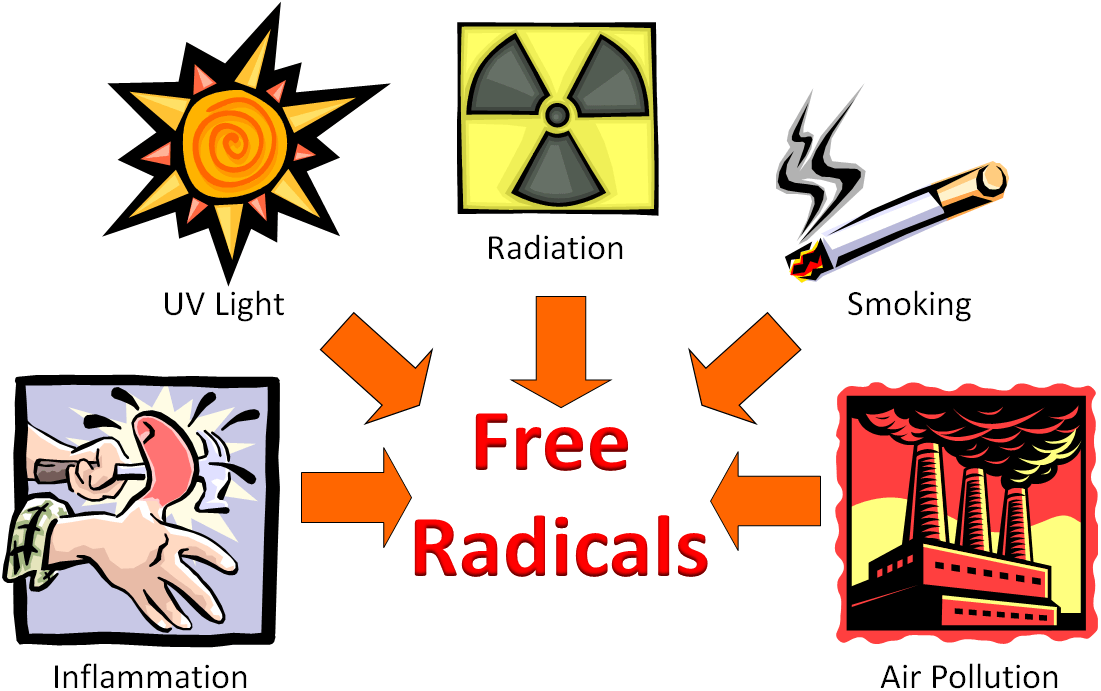
ROS are formed either endogenously as a byproduct of normal metabolic processes in the human body or from other endogenous sources like mitochondria, inflammation, phagocytosis, arachidonate pathways and exercise. These ROS do have roles in cell signalling and homeostasis. Some exogenous sources of free radicals are cigarette smoke, environmental pollutants, radiation (eg UV, X rays), certain drugs (eg drug induced toxicities), pesticides, industrial solvents and ozone.
What is oxidative stress?
A balance between free radicals and antioxidants is necessary for proper physiological function. When ROS overwhelm the cellular antioxidant defence system, whether through an increase in ROS levels or a decrease in the cellular antioxidant capacity, oxidative stress occurs. Short term oxidative stress may occur in tissues injured by trauma, infection, heat injury, toxins and excessive exercise. ROS may have been implicated in carcinogenesis, diabetes mellitus, age-related eye disease, ageing and neurodegenerative diseases such as Parkinson’s disease.
How do free radicals cause damage?
Free radical cascades in the body often terminate when a molecule that loses an electron becomes changed and cannot function without it. This causes damage to the molecule, and thus to the cell that contains it since the molecule often becomes dysfunctional. Free radicals are capable of damaging molecules such as DNA, proteins, carbohydrates and lipids leading to cell and tissue damage. In the skin, this damage over time results in visible signs of ageing including fine lines, wrinkles, discolouration, reduced firmness and even skin cancer.
 What is the free radical theory of ageing?
What is the free radical theory of ageing?The free radical theory of ageing (FRTA) states that organisms age because cells accumulate free radical damage over time. This theory was first proposed by Denham Harman in the 1950s. In 1972 Harman Harman modified his original theory to implicate mitochondria in the production of ROS. This became known as the mitochondrial theory of ageing, which proposes that ROS that are produced in the mitochondria cause damage to lipids, proteins and most importantly mitochondrial DNA.
Since then, the free radical theory has been expanded to include not only ageing but also age related diseases. Free radical damage within ells has been lined to a range of disorders including cancer, arthritis, atherosclerosis, Alzheimers’s disease and diabetes.
 What are antioxidants?
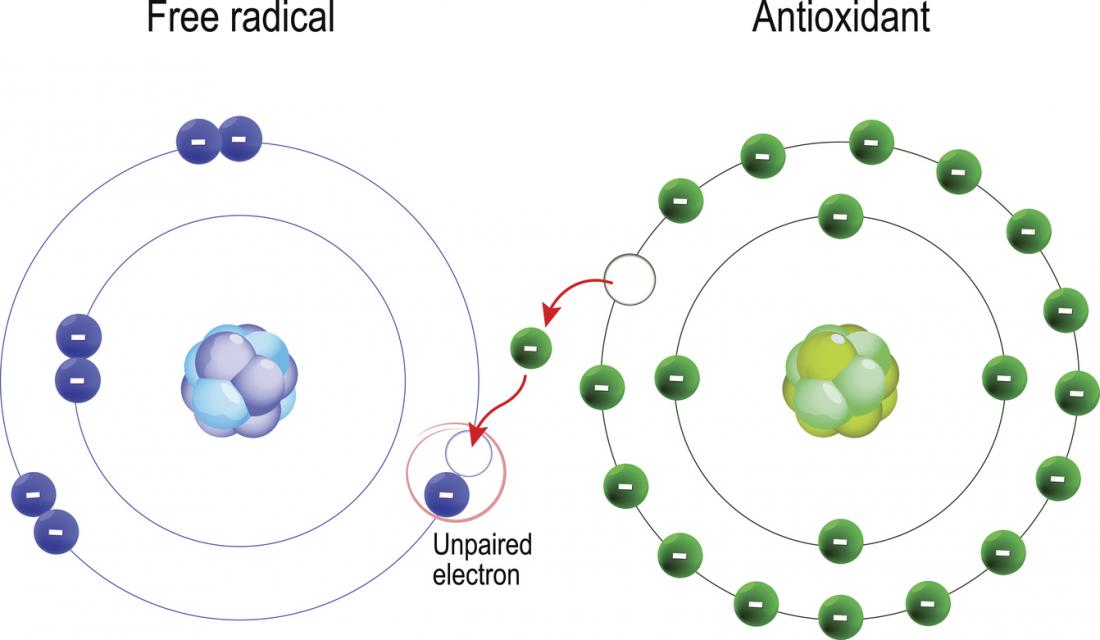
What are antioxidants?Antioxidants are molecules that prevent oxidative reactions by donating an electron to a free radical without becoming destabilised. Antioxidants can safely interact with free radicals and terminate the cascade before vital molecules are damaged. Antioxidants are sometimes referred to as ”free radical scavengers”. Ascorbic acid (Vitamin C), for example, can lose an electron to a free radical and remain stable itself by passing its unstable electron around the antioxidant molecule.
Both enzymatic and non enzymatic antioxidants exist in the intracellular and extracellular environment to detoxify ROS. There are several enzymes within the body that scavenge free radicals such as superoxide dismutase (SOD) catalase and glutathione peroxidase. Some of the non enzymatic antioxidants, including glutathione and ubiquinol are produced during normal metabolism in the body. Other antioxidants must be supplied by diet or in skincare, including vitamin E, vitamin C and B-carotene.
Antioxidants are the skin’s natural way to protect itself from free radicals. However, the effectiveness of the body’s endogenous antioxidant system decreases with age making supplementation essential.